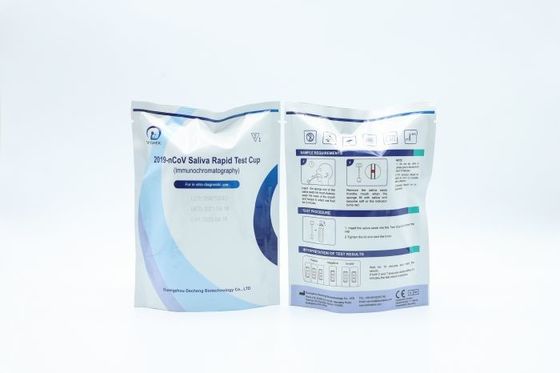
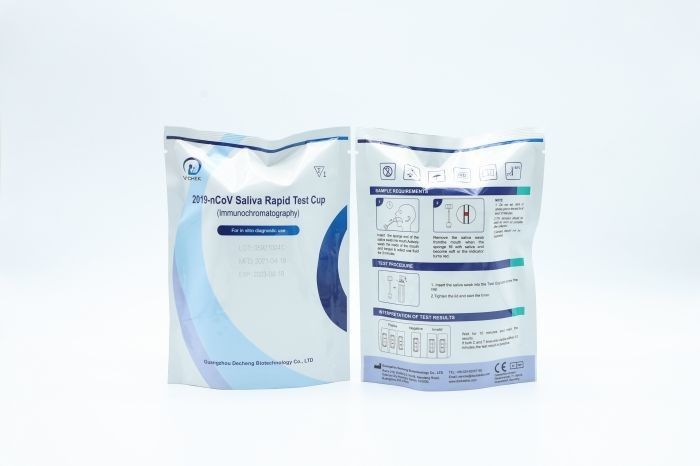
Hospital Rapid Multi Drug Test Cup Male Female Medical Grade test
Product Details:
| Place of Origin: | China |
| Brand Name: | Vchek |
Payment & Shipping Terms:
| Minimum Order Quantity: | negotiable |
|---|---|
| Price: | negotiable |
| Delivery Time: | 10-15days |
| Payment Terms: | T/T, Western Union |
|
Detail Information |
|||
| Sample Type:: | Saliva | Key Words: | 2019 NCoV Ag Saliva Rapid Test Cup |
|---|---|---|---|
| Storage:: | Room Temperature | Accuracy:: | 99.9% |
| Using:: | Self Test | Detection: | Nucleocapsid Protein Antigen From 2019 NCoV |
| High Light: | Hospital Rapid Multi Drug Test Cup,Medical Grade Rapid Multi Drug Test Cup |
||
Product Description
2019 nCoV Ag Saliva Rapid Test Cup
HOW DO LATERAL FLOW TESTS WORK?
Lateral flow tests can be designed to analyse various body fluids, but in the case of COVID-19, most tests analyse material collected from the back of someone’s nose and throat. The swab is then inserted into a tube of liquid, after which a sample of this liquid is deposited on a small absorbent pad contained within the disposable testing kit. The liquid is drawn along the pad by capillary action, until it encounters a strip coated in antibodies which are specific to proteins, also known as antigens, from the SARS-CoV-2 virus. If viral proteins are present, this will show up as a coloured line – much like a positive pregnancy test.
COVID-19 lateral flow tests, also known as COVID-19 rapid diagnostic tests, offer a means of quickly testing for SARS-CoV-2, the virus that causes COVID-19, typically delivering a result in 15-30 minutes. They work in a similar way to home pregnancy tests – except in this case the material being tested comes from a patient's nose and throat, and the kit contains antibodies specific to viral proteins, rather than to a pregnancy hormone.
Features
Rapidly tests for an active COVID-19 infection
Carried out by collecting oral swabs (saliva) - no swab required
Visual, easy to read result in just 15 minutes
98.1% accuracy
High sensitivity and specificity
MATERIALS AND COMPONENTS
Materials provided with the test
| Ingredients | Cassette | Instructions for use | Quick Reference Instructions |
| Specifications | |||
| 0674C4X001 | 1 | 1 | NA |
| 0674C4X002 | 2 | 1 | NA |
| 0674C4X005 | 3 | 1 | 1 |
| 0674C4X010 | 10 | 1 | 1 |
| 0674C4X020 | 20 | 1 | 1 |
| 0674C4X025 | 25 | 1 | 1 |
Materials required but not provided
- Timer
STORAGE AND STABILITY
- Store the test as packaged between 2-30°C.
- The Test stable until the expiration date printed on the outer packing, the product will be expired after 24 months.
- Do not use beyond the expiration date.
- Do not freeze any contents of the test
- The test must remain in the sealed pouch until use.
TEST PROCEDURE
Before test, please read the instructions carefully.
- Take the cassette to equilibrate to room temperature.
- Unpack the aluminum foil bag, take out the cassette.
- Insert the absorber end of the cassette into mouth. Make sure cassette is horizontal statement.
![]()
- Actively swab the inside of the mouth and tongue to collect oral fluid.
- Remove the absorber end from the mouth when the purple color move across the result window in the center of the test device.
- Wait for 10 minutes and read the results.
NOTE:
*When sampling, gently hold it in mouth and let saliva naturally adsorb on the sponge.
*Do not eat, drink, or smoke prior to the test for at least 30 Minutes.
*Any saliva specimen is appropriate for testing but the saliva specimen collected in the morning, before mouth rinsed, eating or drinking, is recommended
INTERPRETATION OF TEST RESULTS
This product can only perform qualitative analysis on the detection object.
Positive Result:
If both C and T lines are visible within 10 minutes, the test result is positive and valid.
Negative Result:
If test area (T line) has no color and the control area displays a colored line, the result is negative and valid
Invalid Result:
The test result is invalid if a colored line does not form in the control region. The sample must be re-tested, using a new test.
![]()
INTERNAL CONTROL
The test contains a built-in internal control in the test card. A color band appearing in the control region (C) is designed as an internal control. The appearance of the control line confirms that sufficient flow has occurred, and that the test card is working normally. If the control line does not appear within 10 minutes, it is considered an error in the test result and it is recommended to test again with the same sample and a new device.
LIMITATIONS
- The result of the test should not be taken as a confirmed diagnosis, for clinical reference only. Judgement should be made along with RT-PCR results, clinical symptoms, epidemiological information, and further clinical data.
- The Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
- The test must be equilibrated to room temperature (18℃~26℃) before used, otherwise the results may be incorrect
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
- React less than 10 minutes may lead a false negative result; React more than 10 minutes may lead a false positive result.
- Positive test results do not rule out co-infections with other pathogens.
- Negative test results are not intended to rule in other viral or bacterial infections.
- Negative results should be treated as presumptive and confirmed with a molecular assay.
- Clinical performance was evaluated with fresh samples.
- Users should test specimens as quickly as possible after specimen collection.
PERFORMANCE CHARACTERISTIC
1. Clinical Verification
The performance of Test was established with 232 sample collected from symptomatic patients, who with symptoms onset within 7 days.
| 2019-nCoV Saliva Ag EASY TEST | Comparative RT-PCR Test Result | ||
| (Immunochromatography) | |||
| Positive (+) | Negative (-) | Total | |
| Detected Positive | 108 | 1 | 109 |
| Detected Negative | 7 | 116 | 123 |
| Total | 115 | 117 | 232 |
| Sensitivity | 93.91%, 95% CI (87.97,97.02) | ||
| Specificity | 99.15%, 95% CI (95.32, 99.85) | ||
| Accuracy | 96.55%, 95% CI (93.34, 98.24) | ||
Positive results broken down by days since symptom onset:
| Days since symptom onset | RT-PCR Positive (+) | 2019-nCoV Saliva Ag EASY CHECK | PPA |
| (Immunochromatography) | |||
| 1 | 13 | 13 | 100% |
| 2 | 32 | 32 | 100% |
| 3 | 52 | 51 | 98.08% |
| 4 | 69 | 67 | 97.10% |
| 5 | 86 | 83 | 96.51% |
| 6 | 102 | 97 | 96.00% |
| 7 | 115 | 108 | 93.91% |
Positive results broken down by CT value:
| 2019-nCoV Saliva Ag EASY TEST | Comparative RT-PCR Method | |
| (Immunochromatography) | (Positive by Ct Value) | |
| Positive (Ct<=25) | Positive (25<Ct) | |
| Detected Positive | 69 | 39 |
| Total | 70 | 45 |
| Positive agreement | 98.57% | 86.67% |
2. Limit of Detection
The experimental results show that for the virus culture concentration above 100 TCID50/mL, the positive rate of detection is greater than or equal to 95%. For the virus culture concentration of 50 TCID50/mL and below, the positive rate of detection is lower than 95%. So, the limit of detection of the Test Card is 100 TCID50/mL.
3. Cross-reactivity
Cross-reactivity of the test card was evaluated. The results showed no cross reactivity with the following specimen.
4. Interference Substances
The test results do not be interfered with the substance at the following concentration:
5. Precision
1. Test 10 replicates of negative and positive by using the reference materials of enterprises. The negative agreement and the positive agreement were 100%.
2. Test three different lots kits including positive and negative reference materials of enterprises. The negative results and the positive results were 100%
6. Hook Effect
The Test Card was tested up to 1.6 × 105 TCID50/ml of heat-inactivated 2019-nCoV strain and no high-dose effect was observed.
PRECAUTIONS
- For in vitro diagnostic use.
- Use appropriate precautions in the collection, handling, storage, and disposal of patient samples and used test contents.
- Use of Nitrile, Latex (or equivalent) gloves is recommended when handling patient samples.
- Do not reuse the used Test Card or saliva swab.
- Should never open the foil pouch of the Test Card exposing it to the ambient environment until the Test Card is ready for immediate use.
- Discard and do not use any damaged or dropped Test Card or material.
- Inadequate or inappropriate sample collection, storage, and transport may yield false test results.
- Sample collection and handling procedures require specific training and guidance.
- To obtain accurate results, do not use visually bloody or overly viscous samples.
- To obtain accurate results, an opened and exposed Test Card should not be used.
- Testing should be performed in an area with adequate ventilation.
- Wear suitable protective clothing, gloves, and eye/face protection when handling the contents of this test.
- Wash hands thoroughly after handling.
KEY TO SYMBOLS USED
| Materials Included | Test Cassette | |||||
| Instructions for Use | Date of | |||||
| Manufacturer | ||||||
| Consult Instructions | Do Not Reuse | |||||
| For Use | ||||||
| Store at 2°C~30°C | Catalogue Number | |||||
| Expiration Date | Keep away from Sunlight | |||||
| Manufacturer | Tests per Kit | |||||
| Lot Number | In Vitro Diagnostic Medical Device | |||||
| Keep Dry | ||||||
| Guangzhou Decheng Biotechnology Co., LTD | ||||||
| Room 218, Building 2, No.68, Nanxiang Road, Science City, Huangpu District, 510000, Guangzhou P.R.China | ||||||
| TEL:+86-020-82557192 | ||||||
| service@dochekbio.com | ||||||
| www.dochekbio.com | ||||||
| CMC Medical Devices & Drugs S.L. | ||||||
| C/ Horacio Lengo Nº 18, CP 29006, | ||||||
| Málaga, Spain | ||||||