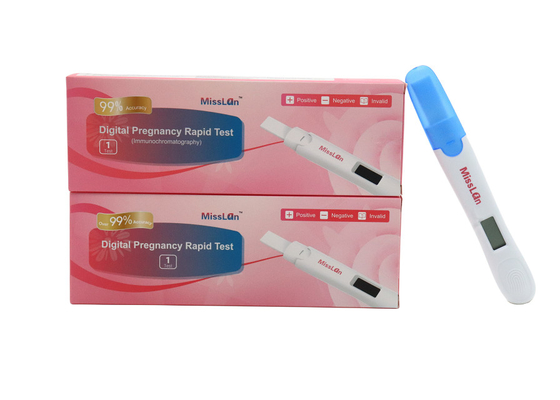
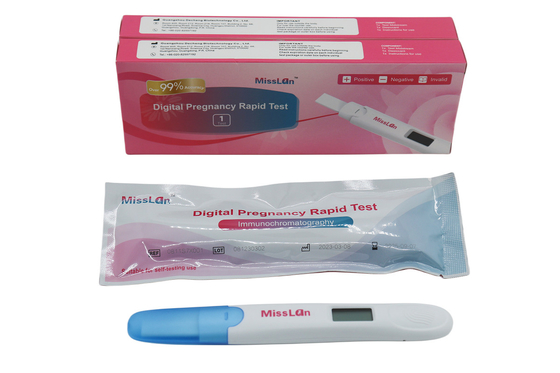
510k CE ANVISA Digital Pregnancy Test Kit OEM 10mIU/mL
Product Details:
| Place of Origin: | China |
| Brand Name: | Miss Lan |
| Certification: | FDA 510k, ANVISA, CE, ISO 13485, MDSAP, BRC |
| Model Number: | DC 811 |
Payment & Shipping Terms:
| Minimum Order Quantity: | 100 |
|---|---|
| Price: | 1.5-2.5 |
| Packaging Details: | 1/2/5 tests per box |
| Delivery Time: | 15-45 days |
| Payment Terms: | T/T, W/U |
| Supply Ability: | 1 million tests/week |
|
Detail Information |
|||
| Detection Level: | 10mIU/mL, 25mIU/mL | Result Accuracy: | 99.9% |
|---|---|---|---|
| OEM/ODM: | Yes | Result Show: | +/-; Pregnant, Not Pregnant |
| Power: | Buil-in Battery | Manufacturer: | Guangzhou Decheng Biotechnology Co., Ltd |
| Warranty: | 2 Years | ||
| High Light: | ANVISA Digital Pregnancy Test Kit,10mIU/mL Digital Pregnancy Test Kit,OEM Digital Pregnancy Test Kit |
||
Product Description
510k/CE/ANVISA Digital Pregnancy Test OEM/ODM Available
Miss Lan Digital Pregnancy Rapid Test
This test device is used for in vitro qualitative detection of Human Chorionic Gonadotropin (HCG) in human urine.
This device is a self-testing IVD medical device for home use.
It is suitable for the early diagnosis of pregnancy.
Test can be done in urine cup or through urine stream.
Test notes
1. The midstream should be used within 1 hour as soon as possible after the foil bag is opened.
2. The first morning urine is the best for more accurate testing.
3. When sample adding is completed, the test midstream will beep.
4. The result will be displayed on the display screen within 5 minutes after adding sample.
5. Do NOT put the absorbent tip of the midstream upwards.
Certifications
Specification
Packing & Delivery
Packing specification
1 test/box
2 tests/box
5 tests/box
Company Profile
Dochek Biotech
As a high-tech company established in 2018, Dochek is dedicated to the R&D, manufacturing and selling of IVD products for rapid test. Analyze equipment and IVD rapid test, in which POCT has become our focus.
So far we have product lines for respiratory system, fertility, DOA, FIA and vet.
We are now exploring into infectious diseases and more home-testing devices
Our purpose is to provide service that can create value of health.
Want to Know more details about this product