Plastic Igm Igg Test Kit One Step 99.9 High Accuracy
Product Details:
| Place of Origin: | China |
| Brand Name: | Vchek |
Payment & Shipping Terms:
| Minimum Order Quantity: | negotiable |
|---|---|
| Price: | negotiable |
| Delivery Time: | 10-15days |
| Payment Terms: | T/T, Western Union |
|
Detail Information |
|||
| Sample Type:: | Saliva | Key Words: | Saliva Antigen Rapid Test Kit |
|---|---|---|---|
| Storage:: | Room Temperature | Accuracy:: | 99.9% |
| Using:: | Self Test | Detection: | Nucleocapsid Protein Antigen From 2019 NCoV |
| Highlight: | 99.9 High Accuracy Plastic Igm Igg Test Kit,Plastic Igm Igg Test Kit One Step 99.9,Igm Igg Test Kit One Step 99.9 Accuracy |
||
Product Description
2019nCov Ag Rapid Test Kit
Size: 25 Test Cassettes
Run Time: <15 min
Sample Type: Serum, Plasma or Whole Blood
Species Sample: Human
Sample Size: 10 µL
Alternative Names: Serology, Coronavirus, COVID-19, Corona, Serological
IVD, CE-Marked
Lancets and Buffer included
Assay Principle
The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is a lateral flow immunochromato-graphic assay. The test uses anti-human IgM antibody (test line IgM) , anti-human IgG (test line IgG) and rabbit IgG (control line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to recombinant COVID-19 antigens conjugat-ed with colloid gold (COVID-19 conjugates). When a specimen followed by assay buffer is added to the sample well, IgM &/or IgG antibodies if present, will bind to COVID- 19 conjugates making antigen antibodies com-plex. This complex migrates through nitrocellulose mem-brane by capillary action. When the complex meets the line of the corresponding immobilized antibody (anti-hu-man IgM &/or anit-human IgG) the complex is trapped forming a burgundy colored band which confirm a reactive test result. Absence of a colored band in the test region indicates a non reactive test result.
To serve as a procedural control, a colored line will always change from blue to red in the control line region, indicat-ing that the proper volume of specimen has been added and membrane wicking has occurred.
PRINCIPLE OF THE TEST
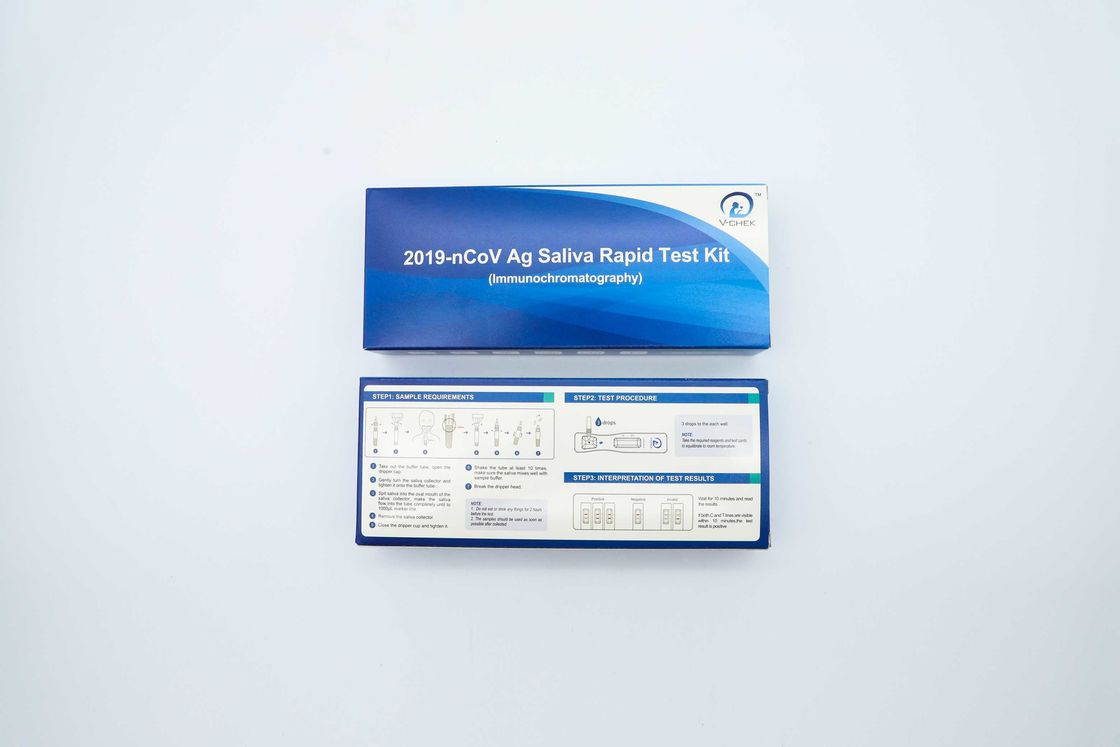
This test uses double-antibody sandwich to legally detect the antigen of novel coronavirus (2019-nCoV) in saliva samples. During detection, the gold labeled anti-2019-nCoV monoclonal antibody in the labeling pad binds to the 2019-nCoV antigen in the sample to form a complex, and the reaction complex moves forward along the nitrocellulose membrane under the action of chromatography, it is captured by the anti-2019-nCoV monoclonal antibody pre-coated by the detection zone (T) on the nitrocellulose membrane, and finally a red color reaction line is formed in the T zone. If the sample does not contain 2019-nCoV antigen, a red color reaction line cannot be formed in the T zone. Regardless of whether the sample to be tested contains 2019-nCoV antigen, a red reaction line will always form in the quality control area (C).
INTENDED USE
This Kit is an in vitro diagnostic test for the qualitative detection of IgG and IgM antibodies to the 2019-nCoV in human serum, plasma, whole blood or finger stick blood.
This product is used as a supplementary detection indicator for suspected cases with negative detection of 2019-nCoV nucleic acid or used in conjunction with nucleic acid detection in the diagnosis of suspected cases.
It cannot be used as a basis for diagnosis and exclusion of 2019-nCoV pneumonia, and is not suitable for general population screening.
A positive test result needs further confirmation. A negative test result cannot rule out the possibility of infection.
This test kit is not for the screening of donated blood.
This test kit is only provided for medical institutions
SUMMARY AND EXPLANATION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
2019-nCoV IgG&IgM Antibody Rapid Test Kit is based on lateral flow immunochromatographic assay. The cassette including: 1) The recombinant novel coronavirus antigen with colloidal gold-labeled and control antibody gold marker; 2) Nitrocellulose membrane with settled two test lines (G and M) and one control line (C). M line with settled monoclonal anti-human IgM antibody; G line with settled reagent for IgG antibody test; C line with settled control antibody. When the proper specimen is added into the test device, it will be absorbed into the device by capillary action, the antibody will combine with 2019-NCOV antigen which colloidal gold-labeled if with the IgM antibody, the immune complex will be captured by the settled anti-human IgM antibody, a colored line appearing in test line(M) which means positive for IgM antibody; and if with the IgG antibody, it will be captured by the settled reagent, a colored line will appearing in test line(G) which means positive for IgG antibody. If test lines G and M without any appearance means negative result. There is a control region C in the cassette, the colored line will appear at the control line C no matter test line appeared or not. To serve as a procedure control, a colored line will appear at the Control Region(C), if the test had been performed properly
MATERIALS AND COMPONENTS
Materials provided with the test kits
|
Specifications
Ingredients |
1 test/Kit |
10 tests/Kit |
20 tests/Kit |
25 tests/Kit |
| Test Cassette | 1 | 10 | 20 | 25 |
|
Sample Buffe 5ml/bottle |
1 | 1 | 1 | 1 |
| Dripper | 1 | 10 | 20 | 25 |
| Instructions for use | 1 | 1 | 1 | 1 |
Note: The components in different batches cannot be used interchangeably.
Materials required but not provided
- Timer clock
- Specimen Collection Containers
- Centrifuge (for serum/plasma sample)
- Appropriate disinfectants.
STORAGE AND STABILITY
- Store at 2°C - 30°C in the sealed pouch up to the expiration date printed on the package. Do not freeze.
- The test cassette is used within 1 hour after taking out from the foil envelope. Buffer solution are re-capped in time after use.
- Keep away from sunlight, moisture and heat.
- Kit contents are stable until the expiration date printed on the outer box.
- The product will be expired after 24 months.
SPECIMEN COLLECTION AND PREPARATION
For whole blood, serum and plasma.
- Using standard phlebotomy procedure, collect a venipuncture whole blood specimen using a blood collection tube with suitable anticoagulant (containing EDTA, Heparin or Citrated sodium). Other anticoagulants have not been validated and may give incorrect result.
- It is recommended that the specimen is tested at the time of specimen collection. If the specimens are not tested immediately, they may be stored at 2°C - 8°C for up to 3 days. Serum or plasma specimens may be stored at -20ºC for up to 9 days. Specimens should not be frozen and thawed repeatedly.
- Frozen specimens must be completely thawed and mixed well prior to testing. Centrifuge the specimen to remove the sediment if with visible particulate matter.
- Severe lipid, hemolysis or turbidity specimens are not recommended
For fingerstick whole blood
- Clean the area to be lanced with an alcohol pad,Massage and/or shake to stimulate blood flow towards the collection area
- Use a sterile lancet, puncture the skin just off the center of the finger pad. Apply gentle pressure beside the point of the puncture. Wipe away the first drop of blood. Create a large drop of blood by applying pressure at the base of the finger and massaging upward.
- Squeeze the pipette bulb to expel air. Draw fingertip blood into the pipet by gently releasing the bulb. The pipet is filled just up to 20ul (second line).
- Whole blood specimen collected by fingerstick are tested immediately.
TEST PROCEDURE
Read the instructions carefully before using. Bring the Detection Cassette, Sample Buffer, and Sample to room temperature before testing
- Allow the cassette, buffer and specimen to equilibrate to room temperature prior to testing.
- Remove a cassette from the foil pouch by tearing at the notch and place it on a level surface.
- Add 10μL of serum, plasma or 20uL fingerstick whole blood, whole blood to the sample hole of the cassette and then add 2-3 drops (80μL) of buffer to the sample hole.
- As the test begins to work, purple color move across the result window in the center of the test device.
- Wait for 15 minutes and read the results. Do not read results after 30 minutes
-
RESULT INTERPRETATION
Positive Result
Both the test line (G) and the control line (C) show color bands, indicating that IgG antibody of the 2019-nCoV is positive; Both the test line (M) and the control line (C) show color bands, indicating that the 2019-nCoV IgM antibody is positive. The test line (M), (G) and control line (C) all show color bands, indicating that the 2019-nCoV IgM and IgG antibodies are positive.
Note: Specimens containing very low levels of target antibodies may develop two colored lines over 15 minutes.
Negative Result
If only the control line C develops color, and neither the G nor M detection lines develop color, no IgM/IgG antibody of 2019-nCoV is detected, and the result is negative.
Invalid Result
The No band appears on the control line (C), and it is judged as an invalid result regardless of whether the detection line (G) (M) shows a band.
PERFORMANCE CHARACTERISTICS
- Sensitivity and Specificity
-
A total of 441 samples were obtained for this study. Prospective specimens include samples from patients who tested positive for 2019-nCoV using nucleic acid tests, samples from patients who recovered from COVID-19. The 2019-nCoV negative specimens include specimens that tested negative by a local hospital.
- H1N1(2009)
- Influenza B
- Rhinovirus type C antibody
- RSV antibody
- Antinuclear antibody
- HIV antibody
- HBV antibody
- HCV antibody
- TP antibody
- Interferences
-
When the sample contains hemoglobin (≤6mg / mL), bilirubin (≤12 mg / dL), triglyceride (≤15 mg / mL), cholesterol (≤10 mg / mL), rheumatoid factor (≤80 IU / mL) at different concentrations, it does not affect the determination of the test results and does not interfere with the test results.
- Precision
-
1. Test 10 replicates of negative by using the reference materials of enterprises, the results are all negative.
2. Test 10 replicates of positive IgM antibody reference materials of enterprise, the results are all positive, and the results of IgG antibody are all negative;
3. Test 10 replicates of positive IgG antibody reference materials of enterprise, the results are all positive, and the results of IgM antibody are all negative.
4, Test three different lots kits including IgM positive, IgG positive, and negative reference materials of enterprises. The negative results and the IgM positive, IgG positive results are 100%.
- Class Specificity
- Reference materials of enterprises for the minimum detection limit of IgM antibody, S1, S2 test results are positive, S3 test results are positive or negative, IgG antibody results are negative;
- Reference materials of enterprises detection limit of IgG antibodies, S4, S5 test results are positive, S6 test results are positive or negative, IgM antibody results are negative.
-
LIMITATIONS OF PROCEDURE
- Negative results do not rule out 2019-nCoV infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnosis are considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude 2019-nCoV infection or to inform infection status.
- Positive results may be due to past or present infection with non-2019-nCoV coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- Coronavirus may not be detected even though coronavirus antibodies are present in the sample, leading to a false negative. This may occur if the amount of coronavirus antibodies is below the detection level of the kit.
- If the product gets wet prior to use, or is stored improperly, it may cause incorrect results.
- This assay is performed as outlined in this manual, and in accordance with all instructions.