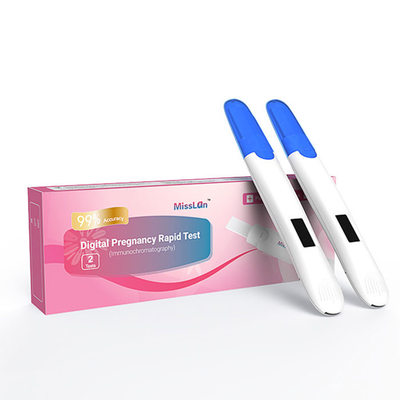
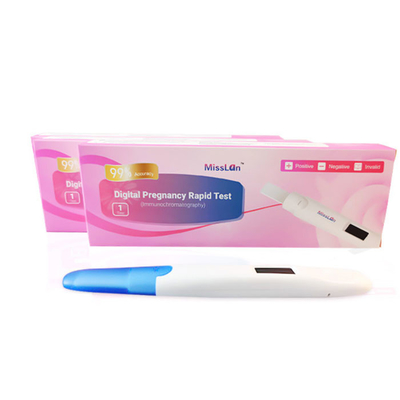
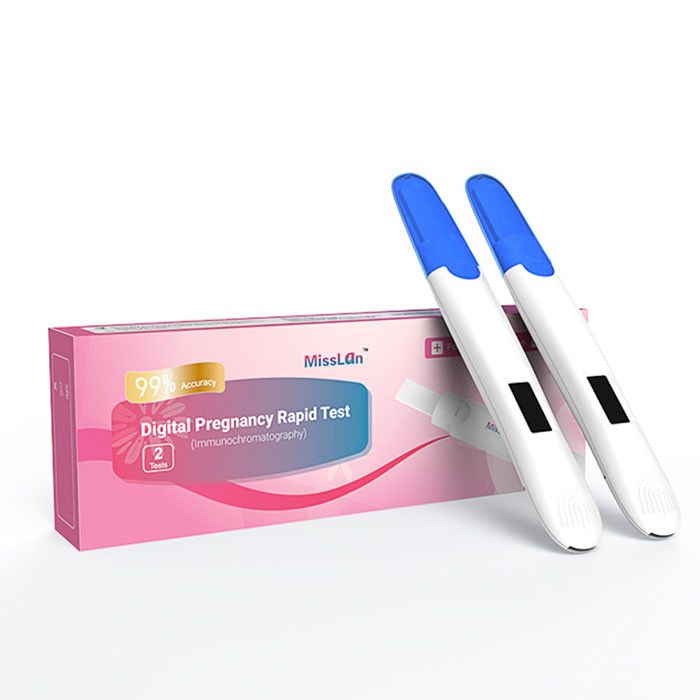
CE Electronic Pregnancy Digital HCG Test Kit Vitro Qualitative Detection
Product Details:
| Place of Origin: | China |
| Brand Name: | MissLan |
| Certification: | CE |
| Model Number: | DC811 |
Payment & Shipping Terms:
| Minimum Order Quantity: | 1000pcs |
|---|---|
| Price: | USD20/TEST |
| Packaging Details: | 1/2test for one box |
| Delivery Time: | 5-8days |
| Payment Terms: | T/T, Western Union |
| Supply Ability: | 3500000test/day |
|
Detail Information |
|||
| Power Source: | Electricity | Shelf Life: | 30months |
|---|---|---|---|
| Test Time: | 5mins | Usage: | Household |
| Function: | Disease Analysis | Warranty: | 2 Years |
| Certificate: | ISO13485 & CE | MOQ: | 1000PCS |
| Package: | 1test/box,2tests/box | Package: | 1test/box,2tests/box |
| High Light: | CE Digital HCG Test Kit,Vitro Digital hCG Test Kit,Guangzhou Electronic Pregnancy Test Bar |
||
Product Description
Electronic Hcg Pregnancy Test, Early Pregnancy Test For Women
Intended Use:
This test device is used for in vitro qualitative detection of Human Chorionic Gonadotropin (HCG) in human urine.
![]()
![]()
![]()
![]()
Specification
| Product name | Digital Pregnancy Rapid Test |
| Method | Immunochromatography |
| Specimen | Urine |
| Accuracy | over 99% |
| Sensitivity | 25mIU/ml |
| Certificate | CE |
| Shelf life | 30months |
| package | 1test/box,2test/box |
1.Store in dry places at 2℃—30℃,do not freeze.
2.The self life is 30 months.Please use the test midstream within 1 hour after opening the foil pouch.
3.See the product package for manufacture date and expiration date.
Dochek was established in 2018, which has Complete platform for the R & D and manufacturing of polyclonal and monoclonal antibodies. As a manufacturer, we dedicated for the IVD Analyze equipment and IVD rapid test technology development, offering total solution of IVD Analyze equipment and IVD rapid test to global market.Specially, our brand “v-chek “rapid test is famous all over the world.
![]()
![]()
![]()
![]()
Q: What is the goods shipping delivery time?
A: After get your payment within 7days.Because our production capacity above 3000000test/day.
Of course, it is no including the new product.