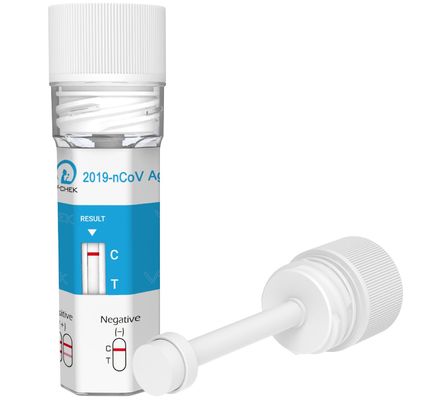
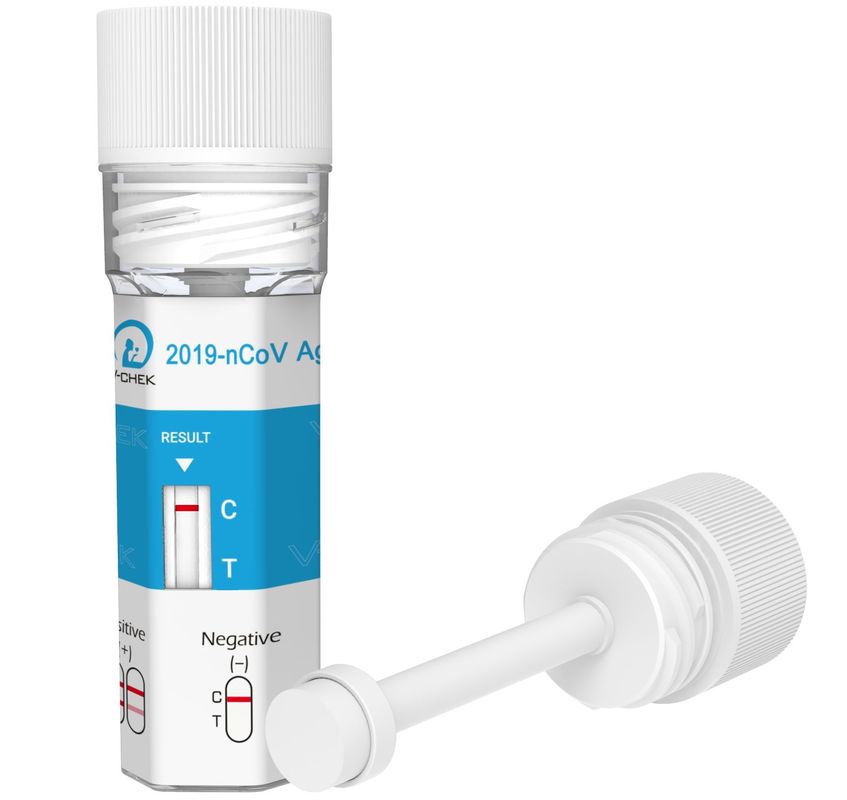
CE Approved 2019-nCoV Ag Saliva Rapid Test Cup For Medical Center
Product Details:
| Place of Origin: | China |
| Brand Name: | Vchek |
| Certification: | CE |
| Model Number: | 0599U4 |
Payment & Shipping Terms:
| Minimum Order Quantity: | negotiable |
|---|---|
| Price: | negotiable |
| Packaging Details: | 1/5/20test for one box |
| Delivery Time: | 7days |
| Payment Terms: | T/T, Western Union |
|
Detail Information |
|||
| Sample Type:: | Saliva | Key Words: | 2019 NCoV Ag Saliva Rapid Test Cup |
|---|---|---|---|
| Storage:: | Room Temperature | Accuracy:: | 99.9% |
| Using:: | Self Test | Detection: | Nucleocapsid Protein Antigen From 2019 NCoV |
| High Light: | CE 2019-nCoV Ag Saliva Rapid Test Cup,One Step COVID 19 Ag Cup |
||
Product Description
2019 nCoV Ag Saliva Rapid Test Cup
Notice
The product is for single use only; do not recycle it. Dispose of used product, samples and other consumables as medical waste in accordance with applicable regulations.
Due to health protection and hygienic reasons, it is not possible to return the goods within 14 days. The seller reserves the right to deliver to the buyer goods of identical manufacturer, the same or better parameters / design (eg due to replacement by a new variant with a different name or production batch).
DESCRIPTION
Rapid test without a nose or throat swab
Apply to the front of the mouth for 90 seconds
Test result after 15 min
Sensitivity: 95.65%
Specificity: 98.44%
suitable for self-testing - even with children
simple application
CE certified
BfArM listed
PRINCIPLE OF THE TEST
This test uses double-antibody sandwich to legally detect the antigen of novel coronavirus (2019-nCoV) in saliva samples. During detection, the gold labeled anti-2019-nCoV monoclonal antibody in the labeling pad binds to the 2019-nCoV antigen in the sample to form a complex, and the reaction complex moves forward along the nitrocellulose membrane under the action of chromatography, it is captured by the anti-2019-nCoV monoclonal antibody pre-coated by the detection zone (T) on the nitrocellulose membrane, and finally a red color reaction line is formed in the T zone. If the sample does not contain 2019-nCoV antigen, a red color reaction line cannot be formed in the T zone. Regardless of whether the sample to be tested contains 2019-nCoV antigen, a red reaction line will always form in the quality control area (C).
MATERIALS AND COMPONENTS
Materials provided with the test
INTENDED USE
The Test Cup is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from 2019-nCoV in saliva specimens directly collected from individuals who are suspected of COVID-19 by their healthcare provider within the first 7 days of symptom onset.
Results are for the identification of 2019-nCoV nucleocapsid protein antigen. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results should be treated as presumptive, and do not rule out 2019-nCoV infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
The Test Cup is intended for use by trained clinical laboratory personnel specifically instructed and trained in vitro diagnostic procedures.
SUMMARY AND EXPLANATION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE OF THE TEST
This reagent uses double-antibody sandwich to legally detect the antigen of novel coronavirus (2019-nCoV) in saliva samples. During detection, the gold labeled anti-2019-nCoV monoclonal antibody in the labeling pad binds to the 2019-nCoV antigen in the sample to form a complex, and the reaction complex moves forward along the nitrocellulose membrane under the action of chromatography, It is captured by the anti-2019-nCoV monoclonal antibody pre-coated by the detection zone (T) on the nitrocellulose membrane, and finally a red color reaction line is formed in the T zone. If the sample does not contain 2019-nCoV antigen, a red color reaction line cannot be formed in the T zone. Regardless of whether the sample to be tested contains 2019-nCoV antigen, a red reaction line will always form in the quality control area (C).
MATERIALS AND COMPONENTS
Materials provided with the test kits
|
Specifications
Ingredients |
0599U4X001 | 0599U4X05 | 0599U4X010 | 0599U4X015 | 0599U4X020 |
| Test Cup | 1 | 5 | 10 | 15 | 20 |
| Saliva Swab | 1 | 5 | 10 | 15 | 20 |
| Instructions for use | 1 | 1 | 1 | 1 | 1 |
| Quick Reference Instructions | NA | 1 | 1 | 1 | 1 |
Note: The components in different batches of the kit cannot be mixed.
Materials required but not provided
- Timer
STORAGE AND STABILITY
- Store the Test Cup as packaged between 2-30°C.
- The Test Cup is stable until the expiration date printed on the outer packing.
- Do not use beyond the expiration date.
- Do not freeze any contents of the test
- The Test Cup must remain in the sealed pouch until use.
SAMPLE REQUIREMENTS
- Insert the sponge end of the saliva swab into mouth. Actively swab the inside of the mouth and tongue to collect oral fluid for 3 minutes.
- Remove the saliva swab from the mouth when the sponge become fully saturated and the inductor turn red.
- Do not eat or drink any things for 4 hours before the test
- The samples should be used as soon as possible after collected.
- Samples should not be inactivated.
![]()
TEST PROCEDURE
Before test, please read the instructions carefully.
- Take the Test Cup to equilibrate to room temperature.
- Unpack the aluminum foil bag, place the Test Cup horizontally on the table and mark it.
- Insert the saliva swab into the Test Cup and screw the cap.
- Tighten the lid and start the timer.
- As the test begins to work, the purple color move across the result window in the center of the test device.
- Wait for 15 minutes and read the results. Do not read results after 20 minutes.
![]()
INTERPRETATION OF TEST RESULTS
This product can only perform qualitative analysis on the detection object.
Positive Result:
If both C and T lines are visible within 15 minutes, the test result is positive and valid.
Note: Specimens containing very low levels of target antibodies may develop two colored lines over 15 minutes.
Negative Result:
If test area (T line) has no color and the control area displays a colored line, the result is negative and valid
Invalid Result:
![]() The test result is invalid if a colored line does not form in the control region. The sample must be re-tested, using a new Test Cup.
The test result is invalid if a colored line does not form in the control region. The sample must be re-tested, using a new Test Cup.
LIMITATIONS
- The result of the Test Cup should not be taken as a confirmed diagnosis, for clinical reference only. Judgement should be made along with RT-PCR results, clinical symptoms, epidemiological information and further clinical data.
- Test Cup performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
- The Test Cup must be equilibrated to room temperature (18℃~26℃) before used, otherwise the results may be incorrect
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
- React less than 15 minutes may lead a false negative result; React more than 15 minutes may lead a false positive result.
- Positive test results do not rule out co-infections with other pathogens.
- Negative test results are not intended to rule in other viral or bacterial infections.
- Negative results should be treated as presumptive and confirmed with a molecular assay.
- Clinical performance was evaluated with fresh samples.
- Users should test specimens as quickly as possible after specimen collection.
PERFORMANCE CHARACTERISTIC
1. Clinical Verification
The performance of Test Cup was established with 232 sample collected from symptomatic patients, who with symptoms onset within 7 days.
Sensitivity: 95.28% (101/106), 95% CI (89.43,97.97)
Specificity: 98.41% (124/126), 95% CI (94.40, 99.56).
| 2019-nCoV Ag Saliva Rapid Test Cup (Immunochromatography) | Comparative RT-PCR Test Result | ||
| Positive (+) | Negative (-) | Total | |
| Detected Positive | 101 | 2 | 103 |
| Detected Negative | 5 | 124 | 129 |
| Total | 106 | 126 | 231 |
The performance of Test Cup with positive results stratified by the comparative method cycle threshold (Ct) counts were collected and assessed to better understand the correlation of assay performance to the cycle threshold, As presented in the table below, the positive agreement of the Test Cup is higher with samples of a Ct count <25.
| 2019-nCoV Ag Saliva Rapid Test Cup (Immunochromatography) |
Comparative RT-PCR Method (Positive by Ct Value) |
|
| Positive>25 (Ct<=25) |
Positive (Ct>25) |
|
| Detected Positive | 80 | 21 |
| Total | 81 | 25 |
| Positive agreement | 98.76% | 84.00% |
2. Limit of Detection
The experimental results show that for the virus culture concentration above 100 TCID50/mL, the positive rate of detection is greater than or equal to 95%. For the virus culture concentration of 50 TCID50/mL and below, the positive rate of detection is lower than 95%. So, the limit of detection of the Test Cup is 100 TCID50/mL.
3. Cross-reactivity
Cross-reactivity of the Test Cup was evaluated. The results showed no cross reactivity with the following specimen.
4. Interference Substances
The test results do not be interfered with the substance at the following concentration:
1. Test 10 replicates of negative and positive by using the reference materials of enterprises. The negative agreement and the positive agreement were 100%.5. Precision
2. Test three different lots kits including positive and negative reference materials of enterprises. The negative results and the positive results were 100%
6. Hook Effect
The Test Cup was tested up to 1.6 × 105 TCID50/ml of heat-inactivated 2019-nCoV strain and no high-dose effect was observed.
PRECAUTIONS
- For in vitro diagnostic use.
- Use appropriate precautions in the collection, handling, storage, and disposal of patient samples and used test contents.
- Use of Nitrile, Latex (or equivalent) gloves is recommended when handling patient samples.
- Do not reuse the used Test Cup or saliva swab.
- Should never open the foil pouch of the Test Cup exposing it to the ambient environment until the Test Cup is ready for immediate use.
- Discard and do not use any damaged or dropped Test Cup or material.
- Inadequate or inappropriate sample collection, storage, and transport may yield false test results.
- Sample collection and handling procedures require specific training and guidance.
- To obtain accurate results, do not use visually bloody or overly viscous samples.
- To obtain accurate results, an opened and exposed Test Cup should not be used.
- Testing should be performed in an area with adequate ventilation.
- Wear suitable protective clothing, gloves, and eye/face protection when handling the contents of this test.
- Wash hands thoroughly after handling.
KEY TO SYMBOLS USED
| Materials Included | Test Cup | ||||
| Instructions for Use | Saliva Swab | ||||
|
Consult Instructions For Use |
Date of Manufacturer |
||||
| Store at 2°C~30°C | Do Not Reuse | ||||
| Expiration Date | Catalogue Number | ||||
| Manufacturer | Keep away from Sunlight | ||||
| Lot Number | Tests per Kit | ||||
| Keep Dry | In Vitro Diagnostic Medical Device | ||||
|
|
Guangzhou Decheng Biotechnology Co., LTD Room 218, Building 2, No.68, Nanxiang Road, Science City, Huangpu District, 510000, Guangzhou P.R.China TEL:+86-020-82557192 sales@dochekbio.com www.dochekbio.com
|
|||
|
CMC Medical Devices & Drugs S.L. C/ Horacio Lengo Nº 18, CP 29006, Málaga, Spain |
||||
Doc No.: DC-IN-0599C01 Ver 1.0
Rel.:2020/12/01